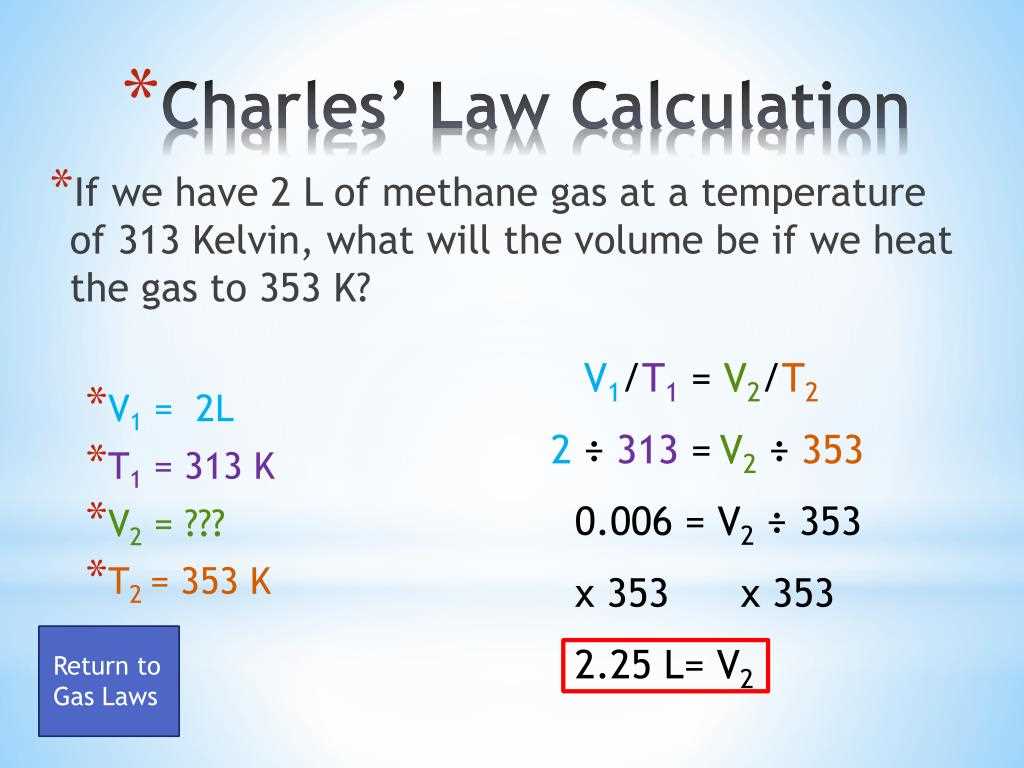
Unlocking the intricacies of the relationship between temperature and volume entails delving into the realm of scientific principles governing the expansion of matter. This exploration navigates through the fundamental tenets of thermal behavior, dissecting the nuanced interplay between heat and the dimensions of substances. By scrutinizing empirical observations and theoretical frameworks, we embark on a journey to comprehend the phenomenon wherein substances undergo alterations in volume as their temperature fluctuates.
Diving deeper into this domain unveils a fascinating narrative of how matter responds to changes in thermal energy, painting a vivid picture of molecular dynamics and interparticle interactions. The narrative intertwines with the fabric of physics, where concepts such as pressure, temperature, and volume converge to elucidate the behavior of gases. Through empirical studies and theoretical formulations, we traverse the landscapes of scientific inquiry, seeking to unravel the mysteries underlying the expansion of gases in response to variations in temperature.
Analyzing the empirical evidence gleaned from meticulous experimentation provides invaluable insights into the empirical regularities governing the expansion of gases. This analysis serves as a cornerstone for understanding the empirical laws that govern the behavior of matter under changing thermal conditions, laying the groundwork for the comprehension of phenomena such as the correlation between temperature and volume, often encapsulated in succinct formulations known as laws.
Understanding the Principles Behind Data Compilation

In this segment, we delve into the core concepts driving the organization of information pertinent to the phenomena associated with Charles’s law. We explore the intricacies of data representation, elucidating the mechanisms through which empirical observations are distilled into comprehensible formats.
Grasping the Essence: At its essence, this section endeavors to encapsulate the fundamental principles underlying the documentation process without explicit reference to the specific nomenclature associated with the law in question. Through careful examination, readers will gain insight into the structured compilation of data, fostering a deeper understanding of the physical phenomena under scrutiny.
Unraveling Patterns: Within these discussions lie explorations into the systematic identification and categorization of variables, as well as the discernment of recurring patterns inherent in experimental data. By shedding light on these processes, we illuminate the pathways through which raw observations metamorphose into organized datasets.
Contextualizing Observations: Furthermore, this segment endeavors to elucidate the significance of contextualizing observations within a broader framework, emphasizing the role of theoretical constructs and empirical evidence in informing the structuring of data. Through such contextualization, the relevance and applicability of recorded phenomena are rendered more palpable.
Synthesizing Insights: Finally, we endeavor to foster a synthesis of insights gleaned from the analysis of data compilation practices, underscoring the iterative nature of scientific inquiry and the imperative of refining conceptual frameworks in light of empirical evidence. Through this synthesis, readers are poised to navigate the intricacies of data interpretation with heightened proficiency and clarity.
The Fundamentals of Proportional Gas Behavior
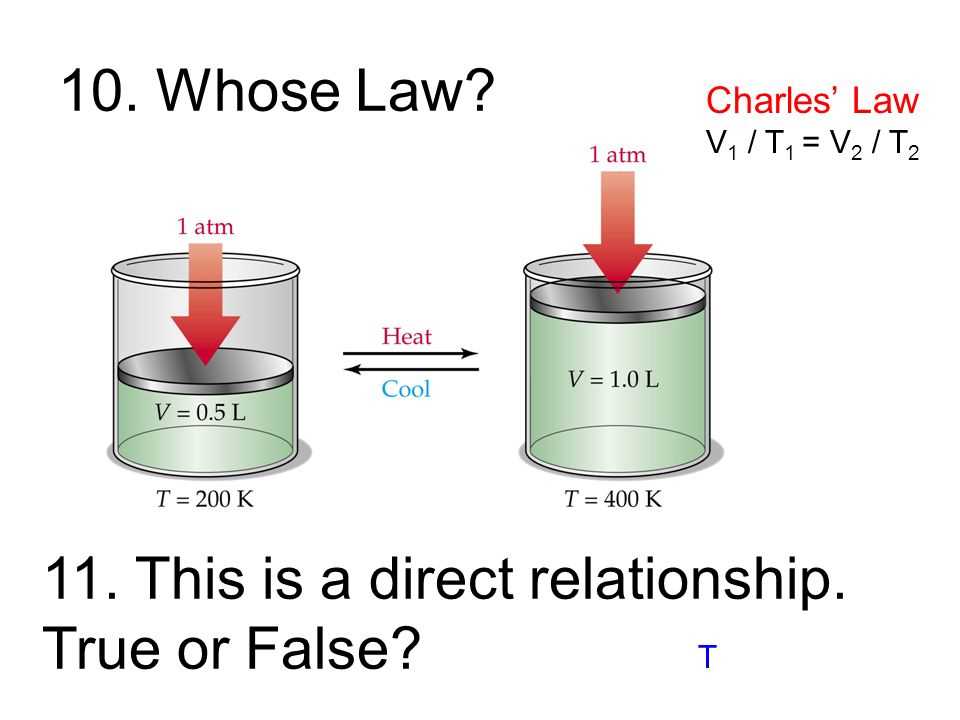
In this section, we delve into the fundamental principles governing the behavior of gases when subjected to changes in temperature and volume, exploring the inherent relationship between these variables. Understanding these principles is essential for grasping the intricacies of gas dynamics.
Temperature and Volume Interplay

At the heart of gas behavior lies a dynamic interplay between temperature and volume, wherein alterations in one parameter induce proportional changes in the other. This fundamental principle, often referred to as thermal expansion in gases, underpins numerous phenomena observed in both natural and controlled environments.
- Temperature Influence: The degree of thermal energy within a gas directly impacts its volume, with an increase in temperature typically resulting in expansion, and conversely, a decrease leading to contraction. This phenomenon is commonly experienced in everyday scenarios, such as the inflation of a balloon when exposed to heat.
- Volume Response: Conversely, alterations in volume exert a reciprocal effect on temperature, manifesting as changes in the kinetic energy of gas molecules. This principle is exemplified in industrial processes like refrigeration, where compression induces a decrease in volume, thereby reducing temperature.
By comprehending these underlying principles, one can navigate the intricacies of gas behavior and apply them effectively in various scientific and practical contexts.
Practical Applications and Examples
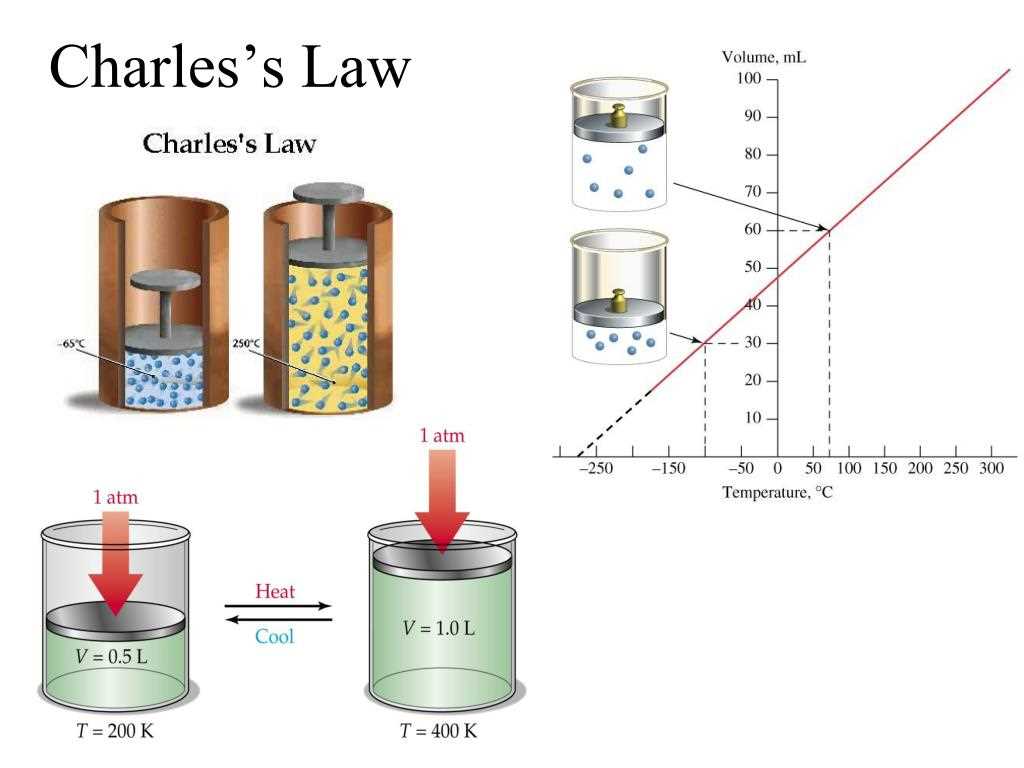
In this section, we delve into real-world scenarios and instances where the principles elucidated by the Charles law find tangible application. Through various illustrations and instances, we explore the practical relevance and utility of the underlying concepts, showcasing their pertinence across diverse fields and contexts.
One notable example pertains to the realm of automotive engineering, where the principles akin to those delineated in the Charles law play a pivotal role in the design and operation of internal combustion engines. By comprehending the behaviors of gases under changing temperatures, engineers can optimize fuel efficiency, enhance performance, and mitigate environmental impacts, thereby contributing to the advancement of sustainable transportation technologies.
Furthermore, the principles encapsulated within the framework of the Charles law find significant utility in the domain of climate control systems. Whether in residential, commercial, or industrial settings, the ability to predict and regulate the expansion and contraction of gases due to temperature variations is paramount for maintaining optimal indoor environments. Such applications underscore the indispensable role of thermodynamic principles in modern-day HVAC (heating, ventilation, and air conditioning) systems.
| Application | Example |
|---|---|
| Chemical Synthesis | In chemical laboratories, precise control over gas volumes at different temperatures is imperative for conducting reactions with accuracy and reproducibility. |
| Weather Balloons | The deployment of weather balloons equipped with sensors relies on an understanding of gas behavior under changing atmospheric conditions, aiding meteorological research and forecasting. |
| Cryogenics | In cryogenic applications, such as the storage and transportation of liquefied gases, adherence to principles akin to those described by the Charles law ensures operational safety and efficiency. |
These examples merely scratch the surface of the myriad practical applications of the principles underlying the Charles law, illustrating its pervasive influence across diverse scientific and technological domains.
Interpreting Gas Volume Behavior in Practical Situations

In the realm of real-world applications, understanding how gas volumes respond to changes in temperature can be pivotal. This section delves into the practical implications of gas volume behavior without explicitly referencing specific scientific principles. Through various scenarios, we explore the dynamic relationship between temperature and gas volumes, shedding light on their interplay in everyday contexts.
| Scenario | Interpretation |
|---|---|
| Hot Air Balloon Ascension | When hot air is introduced into the balloon, the volume of the gas expands, creating buoyancy and causing the balloon to rise. This phenomenon illustrates how temperature influences the volume of the gas, affecting its density and overall behavior. |
| Thermos Flask Insulation | Insulated containers, like thermos flasks, exploit the principle of gas volume behavior to maintain the temperature of liquids. By minimizing heat transfer, these containers effectively control the expansion or contraction of gas volumes within, preserving the desired temperature of the contents. |
| Automotive Tire Pressure | Temperature fluctuations impact tire pressure, as gases inside tires expand in warmer conditions and contract in colder environments. Maintaining optimal tire pressure ensures vehicle safety and performance, highlighting the importance of monitoring gas volume changes with temperature variations. |
These scenarios exemplify the tangible effects of temperature on gas volumes, illustrating how this fundamental concept permeates various aspects of our daily lives. By recognizing and interpreting these behaviors, we gain a deeper appreciation for the role of temperature in shaping the characteristics and behaviors of gases in practical scenarios.